Solving Data Challenges of Genetic and Genomic Testing
Cancer care is full of both complicated nomenclature and the latest buzzwords in healthcare, so it can often be difficult to understand the latest trends. While many of the terms can sound similar, there are differences between genetic and genomic testing or between personalized, preventative, home, and maintenance health plans. The good thing is that all these topics are ones that will improve the quality of care for both cancer patients and the population as a whole.
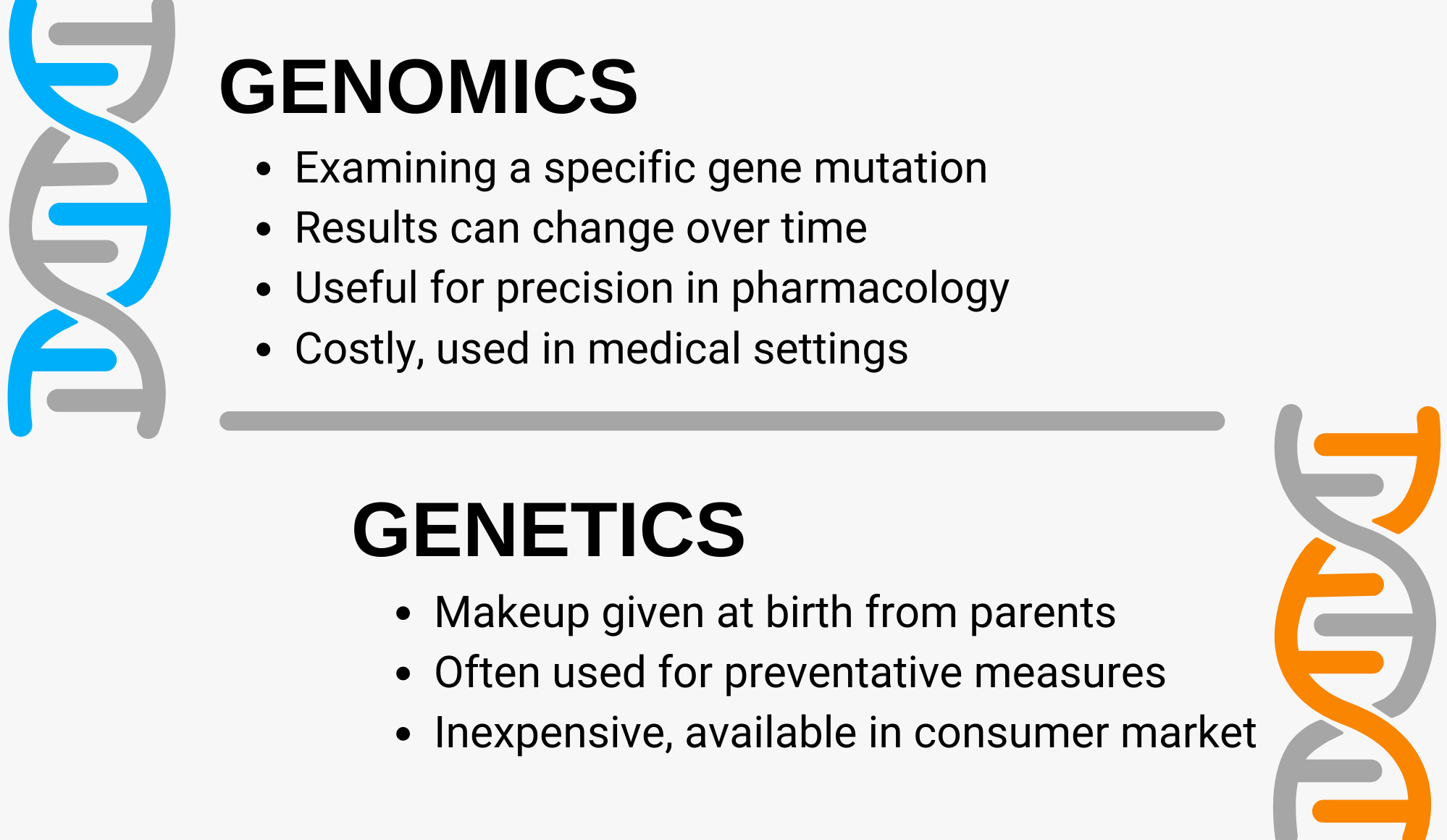
The Difference Between Genetics and Genomics
Genetic testing is an examination of the DNA you’ve inherited from your parents. It’s what we think of from companies like 23andMe, that might offer insight into our heritage, unexpressed traits, or information like a predisposition toward a certain disease. These direct-to-consumer tests have been popularized by their low costs and intriguing ancestry information, but also because of breakthroughs within the medical community. Now those who have a family history of diseases like breast or ovarian cancer can get tested to find out if they have the BRCA1 or BRCA2 gene mutation, giving them advanced knowledge to engage in more prevention and early detection activities.
Given the influx of patients who have received genetic testing results, the healthcare community can benefit from more genetic counselors and educating clinicians as to how to handle patients coming in with these results.
Genomic testing examines mutations in specific genes to assess how they might impact a cancer’s behavior. This type of test is looking at the exceptions to your makeup, the gene abnormality that might be leading to tumor growth. It’s also the type of testing that is conducted over time to monitor further mutations that may occur. It’s particularly important because as mutations occur, treatment efficacies can change, requiring changes for the patient.
These types of medicine adjustments are becoming increasingly common as the field of pharmacogenomics expands. Pharmacogenomics is the practice of taking a patient’s genes into account when administering medicine. To this end, the FDA has been publishing information regarding biomarkers in drug labelling here.
The Data Problems
At cancer centers where genetic and genomic testing is completed in-house, and with patients who are local, data is usually plentiful. Cancer centers are somewhat unique in that their specialization draws patients from a wider geographic area than your average hospital. This means that not only do patients come with large referral packets to sort through, but they also may get genetic or genomic testing done locally, requiring the results to be sent in.
In a perfect world, these results would all be interoperable, but given the impracticality of trying to integrate with everyone sending you results, there are always going to be unstructured documents coming into an organization. For proper trending, analysis, diagnosis, and treatment decisions, the discrete data from these documents must reside in the EHR.
The Answer is Automation
With information this valuable, it’s not enough to have staff simply post results to a patient’s medical record as a PDF. Clinicians working with cancer patients simply don’t have the time to deal with information that’s trapped within an image file. This means that proper discrete data collection and entry falls on the center’s staff.
Automation even drives the research looking for discoveries in oncology. At organizations like Rutgers and Texas Oncology, Extract’s automation software is being used to retrieve information from pathology and genomic reports, capturing hundreds of discrete data fields.
Whether you’re treating patients or researching cures, our software will help you to unlock the complete datasets hiding in non-interfaced documentation. Please reach out today if you’re interested in a demonstration of the software or hearing more about how we’re currently working in oncology.